Building a Spectroscope
Due 4/15/04
In this project we will be building a spectroscope and employing it to observe various types of light sources. Before you begin you will need a pair of scissors, an X-acto knife or a razor blade, standard transparent tape, an opaque type of tape (black electrical tape works well) and black poster board or thin cardboard (eg. from a serial box). A diffraction grating is attached to this document with tape.
Assembly instructions:
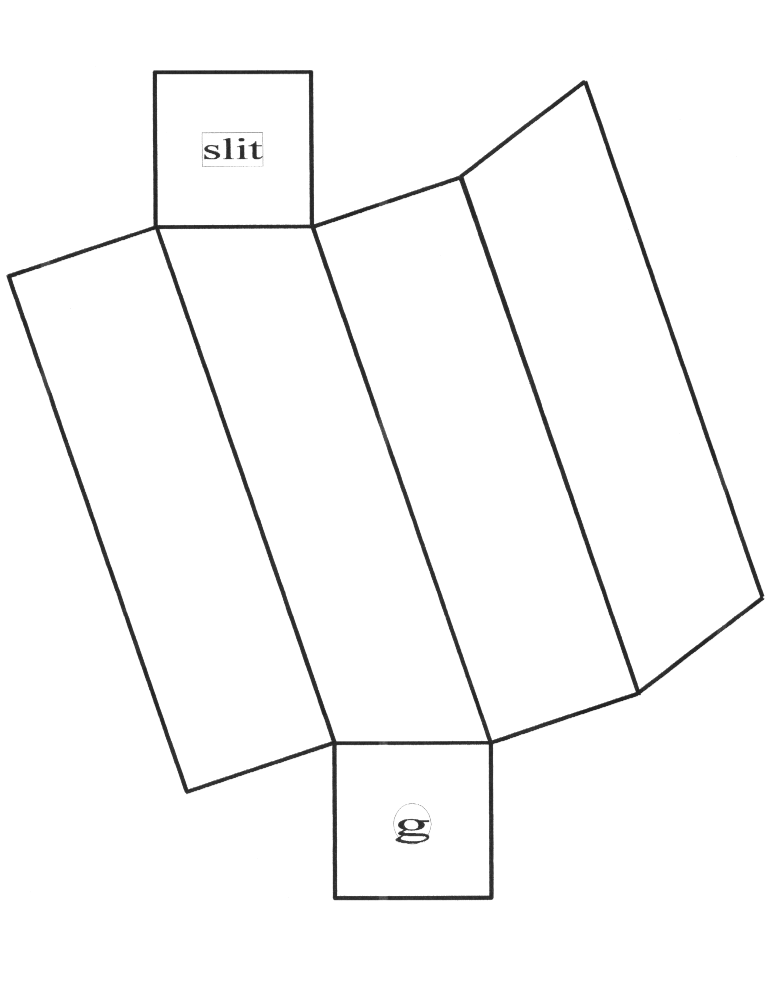
Begin by cutting out the paper template on the last page of this document. Tape it onto the poster board or cardboard using the transparent tape.
Using the X-acto knife, cut the poster board along the outer edge of the template.
Fold the cardboard along the thick black lines to form a square tube. Tape it along the long edge where it meets itself with the black transparent tape. Make this joint as light tight as possible.
Make a circular hole in the end cap marked “g”. Cut a rectangular piece from the diffraction large enough to cover the hole with some room to spare for taping it to the spectroscope. (This should take less than half of the grating.)
Align the grating with the axes of the rectangular end cap. Tape the grating over the hole being careful to not cover the portions of the grating visible through the hole with tape. Fold this end cap up to cover the end of the tube and tape it closed with the opaque tape.
Cut a thin slit (less than 1 mm wide and about 1 cm long) in the center of the other end cap. It's best if the slit is perpendicular to the attached edge of the end cap. Fot this end cap up to cover the end of the tube and seal it with opaque tape.
Point the slit at a light source and look through the diffaction grating you should see several spectra in front of you. The best one should be the one that is most directly in front of you when you put the spectroscope up to your eye. It should be dispersed in the direction perpendicular to the slit.
Contratulations. You've built a spectroscope. Now let's use it.
A standard incandescent light works by heating a filament of tungsten by passing an electrical current through it. It's brightness is determined by the amount of current which determines how hot the filament will get. Use your spectroscope to look at a standard incandescent light bulb. Describe the spectrum. Which end of the spectrum is closer to the slit, red or blue? Is it a continuous spectrum, a line emission spectrum, an absorption spectrum or some combination of the above? Estimate from figures in your book (Ch 6,7) what wavelengths are present in the spectrum. If lines are present give their colors and approximate wavelengths. Make a sketch of the spectrum.
If you have access to an incandescent light with a dimmer switch, describe the difference between the spectrum seen when the switch is at its lowest setting vs the spectrum seen at the highest setting in terms of what colors are present and how bright they are. What explains this difference?
For the next item you will need to find one or more neon signs. A neon sign works by passing an electrical current (a stream of electrons) through a rarified gas. The electrons collide with atoms of the gas electrons in the gas atoms to jump to higher levels. When the electrons jump back down they emit photons. A true neon light (one containing only neon) appears red. Find one and look at it with your spectroscope. Is it a continuous spectrum, a line emission spectrum, an absorption spectrum or some combination of the above? Estimate from figures in your book (Ch 6,7) what wavelengths are present in the spectrum. If lines are present give their approximate wavelengths. Sketch the spectrum.
Find a neon sign of a different color. Signs of differenct colors are made by using different gasses than neon. (Be sure to mention what color the light appears to be.) Is it a continuous spectrum, a line emission spectrum, an absorption spectrum or some combination of the above? Estimate from figures in your book (Ch 6,7) what wavelengths are present in the spectrum. If lines are present give their approximate wavelengths. Make a sketch of the spectrum.
Fluorescent lights operate on a similar mechanism. They contain mercury gas. When a current is passed through mercury gas, it emits mainly in the ultraviolet. The inside of a fluorescent tube is covered with a white solid substance called a phosphor. The phosphor absorbs the ultraviolet and reemits it as visible light. Because the substance is solid, it has many more energy levels than an atomic spectrum. These energy levels are closely grouped together in bands. Look at a fluorescent light with your spectroscope. Estimate from figures in your book (Ch 6,7) what wavelength bands are present in the spectrum. If lines are present give their approximate wavelengths. Sketch the spectrum.
Find at least 3 more types of light to look at. Describe the color that the light appears and describe the spectrum as in each of the above. Which emission process (or combination of emission processes) do you think is responsible for producing the light? What processes, if any, modify the spectrum after the light is produced? Suggestions of light sources to try are: the daytime sky (DO NOT LOOK AT THE SUN WITH YOUR SPECTROSCOPE), the moon, various types of street light, stop and go lights, halogen light bulbs, those new blindingly bright blue car headlights, and anything else you can think of.
